Exploring the Hydrogen Atom: The Fundamental Building Block of the Universe
The hydrogen atom is not just the simplest of all atoms; it holds profound significance in physics, chemistry, and cosmology. Its unique structure and properties have captivated scientists for centuries, and its presence is integral to understanding the fabric of the universe. In this article, we delve into the hydrogen atom’s properties, structure, historical discoveries, and its pivotal role in various scientific domains.
Understanding the Hydrogen Atom: Structure and Properties
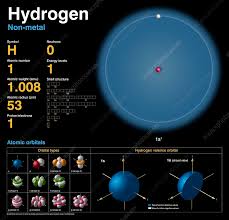
The hydrogen atom is the lightest and most abundant element in the universe, consisting of a single proton in its nucleus and a single electron bound to it. This seemingly simple configuration forms the basis for a rich array of physical phenomena.
Atomic Number and Mass: Hydrogen has an atomic number of 1, indicating one proton in the nucleus. It has an atomic mass of approximately 1.008 unified atomic mass units (u), which accounts for isotopic variations.
Electron Configuration: The sole electron in a hydrogen atom occupies the 1s orbital in its ground state, the closest orbital to the nucleus.
Isotopes of Hydrogen:
Protium (ⁱH): The most common isotope, consisting of one proton and no neutrons.
Deuterium (²H): A stable isotope containing one neutron, used in nuclear fusion research and tracing chemical reactions.
Tritium (³H): A radioactive isotope with two neutrons, employed in scientific experiments and as a fuel source in proposed fusion reactors.
Hydrogen Molecules: Most hydrogen exists as diatomic molecules (H₂) under standard conditions. Molecular hydrogen is stable, colorless, odorless, and highly flammable.
Historical Significance: From Early Theories to Quantum Mechanics

The journey of understanding the hydrogen atom reflects humanity’s evolving comprehension of matter:
Pre-modern Views: Ancient Greek philosophers, such as Democritus, postulated the existence of atoms as the indivisible building blocks of matter. However, their ideas lacked experimental verification.
Discovery of Hydrogen:
Henry Cavendish (1766) was the first to recognize hydrogen as a distinct element, describing it as “inflammable air.”
Hydrogen was later named by Antoine Lavoisier, derived from the Greek words hydro (water) and genes (creator).
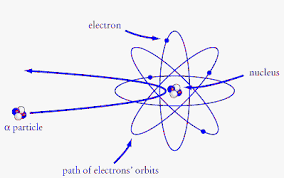
Rutherford’s Model (1911): Ernest Rutherford’s gold foil experiment revealed that atoms consist of a dense nucleus surrounded by electrons. This laid the groundwork for understanding the hydrogen atom’s structure.
Bohr Model (1913): Niels Bohr introduced a quantum model for the hydrogen atom. He proposed that electrons orbit the nucleus in discrete energy levels, emitting or absorbing energy as they transition between levels.
Modern Quantum Mechanics:
Schrödinger’s equation provided a wave function (ψ) describing the electron’s probable location.
The hydrogen atom became a testing ground for quantum theory, leading to the development of advanced concepts like quantum numbers and orbital shapes.
Hydrogen in Astrophysics and Cosmology
Hydrogen’s ubiquity and simplicity make it a cornerstone of astrophysics. Here are some of its key roles:
Primordial Hydrogen: Formed approximately 380,000 years after the Big Bang, hydrogen represents the first element to emerge in the universe.
Fuel for Stars: Hydrogen undergoes nuclear fusion in stars, combining to form helium and releasing immense energy. This process powers stars like our Sun and creates heavier elements via nucleosynthesis.
21-Centimeter Line: Neutral hydrogen emits electromagnetic radiation at a wavelength of 21 cm, crucial for mapping the distribution of hydrogen clouds in galaxies.
Role in Exoplanet Atmospheres: Hydrogen’s presence in the atmospheres of exoplanets can provide clues about their composition and potential habitability.
Hydrogen in Chemistry and Industrial Applications
Hydrogen’s chemical properties and abundance make it indispensable across various domains:
Bonding and Compounds:
Covalent Bonds: Hydrogen forms covalent bonds in water (H₂O) and organic molecules like hydrocarbons.
Hydrides: Compounds like lithium hydride (LiH) and sodium hydride (NaH) exhibit ionic bonds with hydrogen.
Industrial Uses:
Ammonia Production: Hydrogen is a key component in the Haber-Bosch process, producing ammonia (NH₃) for fertilizers.
Hydrogenation Reactions: Widely used in the food industry to convert unsaturated fats to saturated fats.
Energy Carrier:
Hydrogen Fuel Cells: Hydrogen reacts with oxygen in fuel cells to produce electricity, emitting only water vapor as a byproduct.
Green Hydrogen: Produced via electrolysis powered by renewable energy sources, it is a promising candidate for sustainable energy solutions.
Hydrogen’s Quantum Mechanics: Theoretical Insights
The hydrogen atom has been pivotal in advancing quantum theory. Key contributions include:
Wave-Particle Duality: Hydrogen’s spectral lines supported the notion of light and electrons exhibiting wave-particle duality.
Quantum Numbers:
Principal quantum number (∞n): Indicates the energy level.
Angular momentum (ℓ): Describes orbital shape.
Magnetic quantum number (m): Specifies orientation.
Spin (±½): Accounts for the electron’s intrinsic angular momentum.
Hydrogen Spectroscopy: The Balmer and Lyman series are foundational in understanding electron transitions and electromagnetic spectra.
Future Directions: Hydrogen and Sustainability
Hydrogen’s potential for enabling a sustainable future is immense:
Clean Energy Revolution:
Transitioning from fossil fuels to hydrogen-based energy systems could significantly reduce greenhouse gas emissions.
Advancements in Hydrogen Storage: Challenges such as low volumetric energy density are being addressed through innovations like metal hydrides and liquid organic hydrogen carriers.
Hydrogen in Space Exploration: As a lightweight and energy-dense fuel, hydrogen powers rockets and could play a critical role in future extraterrestrial missions.
Conclusion
The hydrogen atom, while simple in structure, is profoundly complex in its implications for science and society. From elucidating fundamental principles of quantum mechanics to driving the quest for sustainable energy, hydrogen is truly the building block of progress. Its study continues to open new frontiers, fostering our understanding of the universe and paving the way for a greener, more connected future.